Introduction
Interferons (IFN) are signaling proteins that help the body fight infections, in part by priming antiviral immunity. However, they seemed to play a paradoxical role in COVID-19. Pre-existing defects of FN responses were associated with adverse prognosis, yet giving IFN therapeutically did not improve COVID-19 outcomes, and may have worsened severe disease. Furthermore, severe COVID-19 was characterized by very high levels of IFN. Since IFN are involved in a number of infections, it’s important to understand how it impacts the immune response, so that we know how it should be modulated.
To explore these questions, we run an integrated analysis of blood molecular signature from hospitalized COVID-19 positive patients. Our results reveal 4 distinct subgroups, underlining the heterogeneity of the immune response to the virus. Particularly, we identify a cluster of high-risk patients, in which exacerbated inflammatory responses correlate with poor survival outcomes. We further show how higher IFN is associated with a delayed antibody response throughout the cohort, suggesting a mechanism for the Interferon Paradox.
Development
We investigate 242 enrolled hospitalized COVID-19+ patients with symptomatic infection from two hospitals in Montreal, Quebec, Canada and replicate parts of our analysis on data from 76 patients from Uppsala, Sweden. Most studies group patients based on observable clinical characteristics across the duration of their hospitalization, which can blur our understanding of highly dynamic diseases like COVID-19. We instead group patients using the k-means algorithms on blood molecular signatures around 11 days after symptoms began. Central to our analysis is the PHATE algorithm, which allows us to project molecular profiles in 2D to visualize how “far apart” patients and groups are in the data space.
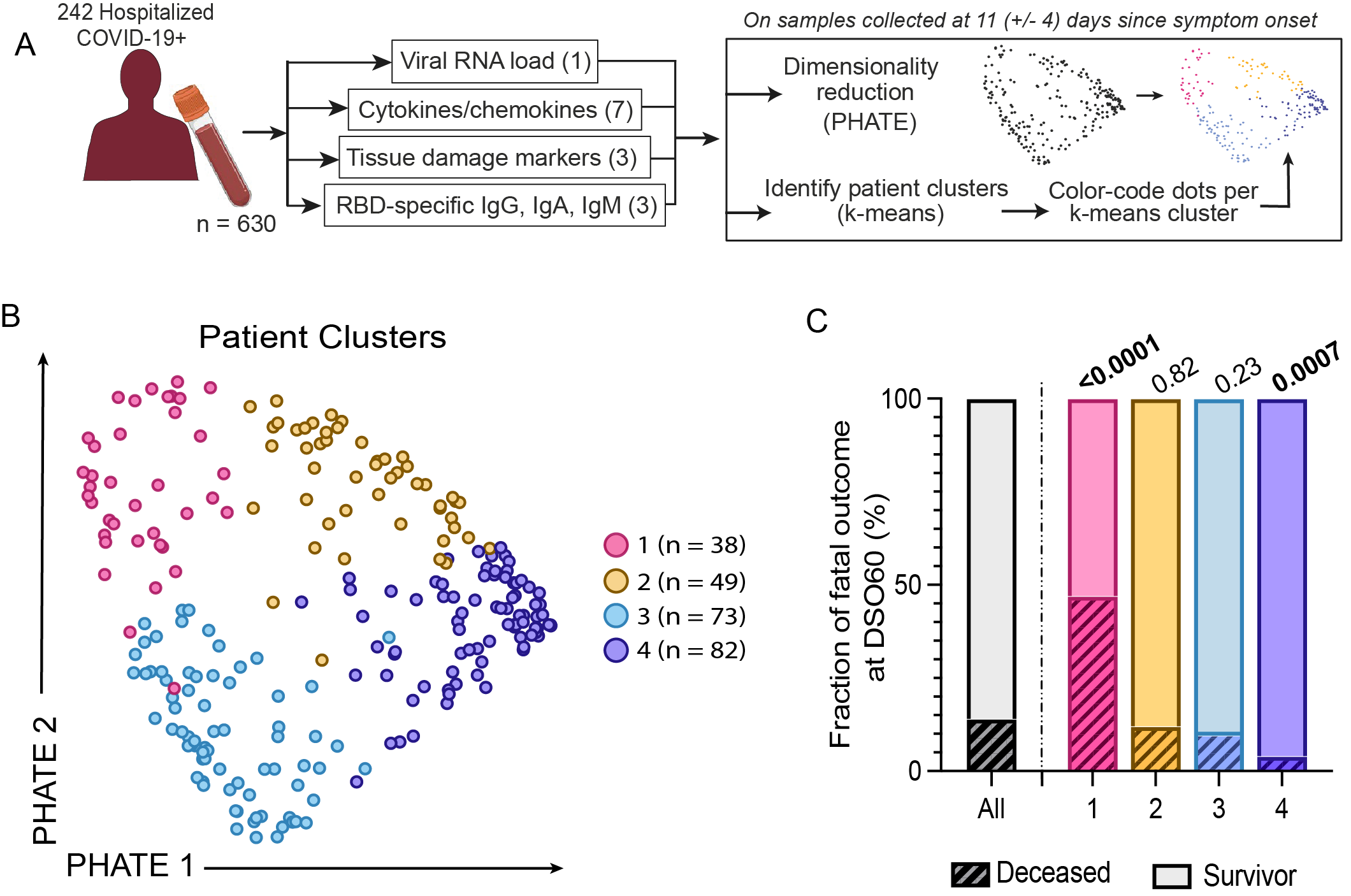
Our integrated analysis revealed 4 distinct patient profiles with different immune responses to the virus. The first two groups correspond to critical cases requiring mechanical ventilation, with one group particularly enriched in fatal outcomes. This high-fatality group exhibits distinct quantities of viral particles, antibodies and tissue damage markers. The two other groups are non-critical survivors, separated by the timing of their antibody response. Our patient characterization not only aligns with disease severity, but also delineates survival probability among critical cases. Some of our findings are also reflected in the PHATE visualization: the left-right axis aligns with the timing of the antibody response, while the top-down axis correlates with viral load and other markers of disease severity.
We wanted to understand the delay in antibody generation in the high-fatality group and one of the non-critical groups, so we looked at what genes were expressed in each group at 11 days after symptom onset. Those with the delay had higher signatures of IFN, which also had a negative correlation with other immune responses.
Conclusion
To understand the range of disease severity from people in hospital from COVID-19, we sampled the blood of over 300 individuals at a specific time in their disease course, and measured a number of markers related to their immune response and the infection. We saw four “types” of patients : two non-critical groups, either with a delay or not in their antibody response and who both did well, and two critical groups, with one of them encompassing nearly all of the COVID-19-associated deaths. This high fatality group also had a delay in their antibody response. Both groups with this delay had high IFN for longer, which was also associated with delays in other immune responses. This was the first in-human characterization of the “IFN paradox” (previously only seen in mice and monkeys), where the quantity and the timing of the IFN response are key in having a proper immune response to a viral infection. This study explains the seemingly conflicting evidence around IFN in COVID-19, and has implications for treatment of COVID, but also perhaps of other viral infections.
References
Brunet-Ratnasingham, Elsa, et al. "Sustained IFN signaling is associated with delayed development of SARS-CoV-2-specific immunity." Nature Communications 15.1 (2024): 4177.