Improving medical image analysis with AI
Using deep learning to predict individual outcome and response to treatment for patients with neurological diseases.
Using deep learning to predict individual outcome and response to treatment for patients with neurological diseases.
Personalized medicine tailors treatment to the individual patient, and can be particularly crucial in chronic diseases such as multiple sclerosis (MS), where timely and effective treatment is essential to prevent morbidity and reduce disability.
While current decisions are based on general statistics and population-based clinical markers, harnessing patient images for personalized predictions can enable individualized treatment recommendations that better account for variability in drug efficacy across patients.
Yet these models remain underexplored, and the open challenges presented by real-world clinical settings hamper their safe deployment.
We develop deep learning models leveraging medical images for real-world clinical contexts and improved patient care. This could result in tools to assist clinicians with the selection of optimal treatments for individual patients with chronic, incurable disease, as well as improved clinical trial analysis.
We seek to develop modern deep learning frameworks which accurately predict future patient outcomes and individual treatment effects, requiring them to overcome challenges presented by real-world data.
We also need to ensure the safety and reliability of the models for successful clinical deployment.
In particular, this model can accurately predict the future worsening of a patient's disease under the effect of all possible therapies (and a placebo). The aim was also to identify individuals likely to respond to different treatments in heterogeneous populations, by predicting future individual treatment effects. At the individual level, this can be used to improve treatment recommendations: the framework helped identify sub-populations of patients who responded to treatments.
We are striving to improve model safety and reliability by building the first uncertainty-aware causal models for imaging-based personalized medicine. Communicating the probability of response to treatment would enable more informed recommendations to be made.
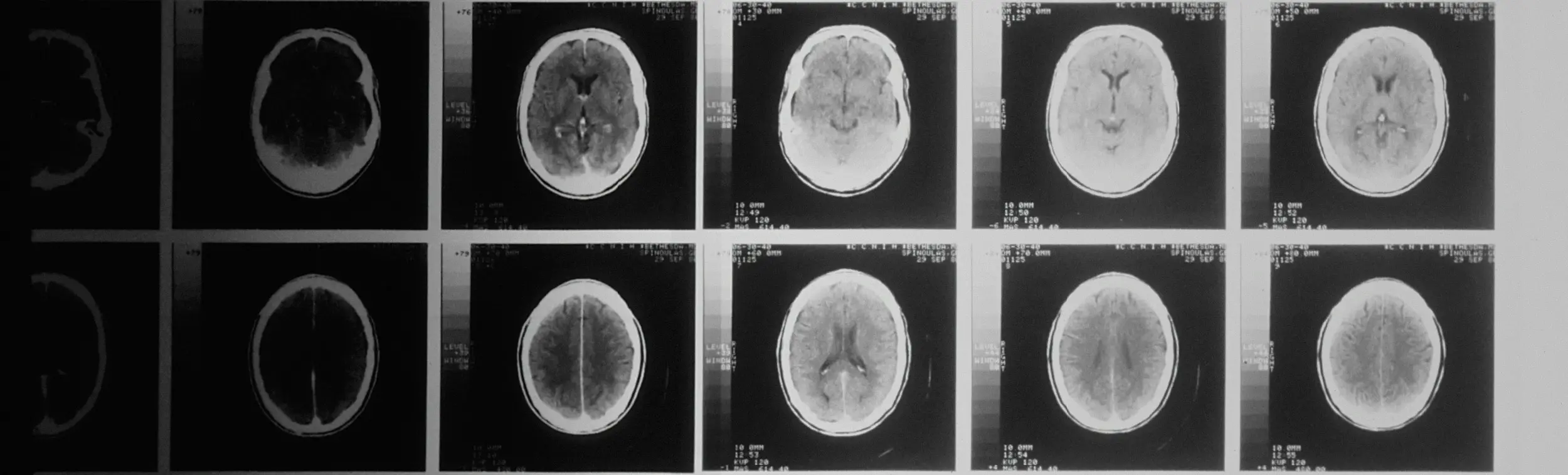
We tested the model on a large clinical trial dataset of multiple sclerosis patients, provided by the International Progressive MS Alliance.
NeurologyLive spoke to Tal Arbel, Mila Associate Academic Member, at the ACTRIMS forum to find out more about the work being done in this field.
Joshua Durso-Finley (McGill/Mila) speaks at the USS (UNIQUE Student Symposium) in a session on NeuroAI in medical research.

I'm proud to be developing AI tools to improve outcomes for patients with long-term incurable diseases, together with my incredible research team.
+1,800
Over 1,800 MS patients have been analyzed as part of the project.
5
Experiments were conducted on 5 different treatments.
4
The model was tested in 4 randomized clinical trials.